Cardiff Oncology Announces Positive Data from Ongoing Randomized Phase 2 First-line RAS-mutated mCRC Clinical Trial (CRDF-004)
– Trial demonstrates 49% confirmed ORR in the 30mg onvansertib dose arm versus 30% confirmed ORR in the control arm in intent-to-treat population (N=110) –
– Early PFS data show a trend favoring 30mg onvansertib dose arm vs. control arm –
– Onvansertib continues to be well-tolerated and demonstrates a dose dependent response for all endpoints including ORR, early tumor shrinkage and depth of response –
– Company will hold a conference call today at 4:30 p.m. ET / 1:30 p.m. PT –
SAN DIEGO, July 29, 2025 (GLOBE NEWSWIRE) -- Cardiff Oncology, Inc. (Nasdaq: CRDF), a clinical-stage biotechnology company leveraging PLK1 inhibition to develop novel therapies across a range of cancers, today announced positive data from the ongoing CRDF-004, a randomized, Phase 2 clinical trial evaluating onvansertib in combination with standard-of-care (SoC) in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). Efficacy data represents intent-to-treat patients as of a July 8, 2025 data cut-off, and is determined by blinded, independent central review (BICR) of each patient’s tumor scans.
“We are highly encouraged by the 19% improvement in confirmed ORR as well as the shorter time to response and deeper tumor regression observed in our trial with onvansertib combined with SoC compared to SoC alone. Furthermore, early PFS data shows a trend favoring the 30mg dose of onvansertib vs. control,” said Roger Sidhu, MD, Chief Medical Officer of Cardiff Oncology. “The totality of the data we are releasing today strengthens the initial findings from our December 2024 data release in a significantly larger patient population, compares favorably to previous practice-changing Phase 3 trials, and demonstrates that onvansertib could be a novel therapy for the treatment of first-line RAS-mutated mCRC.”
Trial Design
The CRDF-004 phase 2 trial enrolled patients with mCRC who have a documented KRAS or NRAS mutation. Onvansertib is added to SoC consisting of FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab. Patients were randomized to one of six arms including 20mg of onvansertib plus SoC, 30mg of onvansertib plus SoC, or SoC alone. The primary endpoint is objective response rate (ORR), and the secondary endpoints include progression-free survival (PFS), duration of response (DOR) and safety. Additional prespecified endpoints include early tumor shrinkage (ETS), defined as a ≥20% reduction in tumor size at the 2-month scan, and depth of response (DpR), defined as the greatest reduction in tumor size achieved while on therapy during the trial. ETS and DpR are well-established predictors for PFS in first-line mCRC as demonstrated in multiple peer-reviewed publications.1-3
Efficacy Data
Efficacy data in the intent-to-treat population (ITT) from the CRDF-004 clinical trial, as of the July 8, 2025 data cut-off, are shown below.
|
Control Arm (SoC alone) (n=37) |
20mg dose of onvansertib + SoC (n=36) |
30mg dose of onvansertib + SoC (n=37) |
|
| Confirmed ORRa | 30% (11 of 37) |
42% (15 of 36) |
49% (18 of 37) |
| Confirmed ORR at 6-monthsa |
22% (8 of 37) |
33% (12 of 36) |
46% (17 of 37) |
| ORRb | 43% (16 of 37) |
50% (18 of 36) |
59% (22 of 37) |
aConfirmed Objective Response Rate (ORR) per RECIST v1.1 includes those patients who had a complete response (CR) or partial response (PR) confirmed by repeat imaging ≥4 weeks after response criteria first met. bORR per RECIST v1.1 includes confirmed CRs/PRs and unconfirmed PRs who were still on treatment and may yet be confirmed
Spider Plots, displaying the change in tumor size from baseline for each patient over time, demonstrate deeper responses in patients receiving the 30mg dose of onvansertib in combination with the SoC compared to both the control arm and 20mg dose of onvansertib arm.
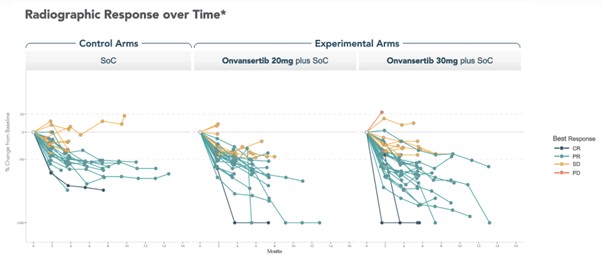
Note: Radiographic response was determined per RECIST 1.1 by blinded independent central review. Spider plot reflects data as of July 8, 2025 from an ongoing trial and unlocked database.
Progression-free Survival (PFS) Data
Both the 20mg and 30mg onvansertib arms demonstrated an early separation of the PFS curves compared to the control arm at a median follow up time of 6 months. While the median PFS has not been reached, there was a dose dependent effect in favor of the 30mg onvansertib dose.
Safety and Tolerability
The safety analysis was conducted for the 104 patients who were dosed in the trial. Onvansertib in combination with chemo/bevacizumab was well-tolerated and there were no major or unexpected toxicities observed. Grade 3 or higher adverse events were infrequent, with neutropenia being the most common treatment-emergent adverse event associated with onvansertib.
“We are highly encouraged by the strength of our data which achieves the key objectives we set for the trial, and positions us to engage in discussions with the FDA as we advance toward our registrational CRDF-005 trial,” said Mark Erlander, Chief Executive Officer of Cardiff Oncology. “Looking ahead, we are optimistic about onvansertib’s potential to redefine the first-line treatment for RAS-mutated mCRC and will provide an update on our first-line mCRC program by Q1 2026.”
Upcoming expected milestones
- Update on first-line mCRC program expected by 1Q 2026
Conference Call and Webcast
Cardiff Oncology will host a conference call and live webcast at 4:30 p.m. ET / 1:30 p.m. PT on July 29, 2025. Individuals interested in listening to the live conference call may do so by using the webcast link in the "Events" section of the company's website. A webcast replay will be available in the investor relations section on the company's website following the completion of the call.
About Cardiff Oncology, Inc.
Cardiff Oncology is a clinical-stage biotechnology company leveraging PLK1 inhibition, a well-validated oncology drug target, to develop novel therapies across a range of cancers. The Company's lead asset is onvansertib, a PLK1 inhibitor being evaluated in combination with standard of care (SoC) therapeutics in clinical programs targeting indications such as RAS-mutated metastatic colorectal cancer (mCRC), as well as in ongoing and planned investigator-initiated trials in metastatic pancreatic ductal adenocarcinoma (mPDAC), small cell lung cancer (SCLC) and triple negative breast cancer (TNBC). These programs and the Company's broader development strategy are designed to target tumor vulnerabilities in order to overcome treatment resistance and deliver superior clinical benefit compared to the SoC alone. For more information, please visit https://www.cardiffoncology.com.
References
- Cremolini, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188–1194. doi: 10.1093/annonc/mdv112
- Piessevaux, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013 Oct 20;31(30):3764-75. doi: 10.1200/JCO.2012.42.8532
- Bando H, et al. Associations between early tumor shrinkage/depth of response and survival from the ARCAD database. JNCI Cancer Spectr. 2025 Apr 30;9(3):pkaf042. doi: 10.1093/jncics/pkaf042
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified using words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Cardiff Oncology's expectations, strategy, plans or intentions. These forward-looking statements are based on Cardiff Oncology's current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidate; results of preclinical studies or clinical trials for our product candidate could be unfavorable or delayed; our need for additional financing; risks related to business interruptions, including the outbreak of COVID-19 coronavirus and cyber-attacks on our information technology infrastructure, which could seriously harm our financial condition and increase our costs and expenses; uncertainties of government or third party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that our product candidate will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that our product candidate will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in Cardiff Oncology's Form 10-K for the year ended December 31, 2024, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Cardiff Oncology does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
Cardiff Oncology Contact:
James Levine
Chief Financial Officer
858-952-7670
jlevine@cardiffoncology.com
Investor Contact:
Kiki Patel, PharmD
Gilmartin Group
332-895-3225
Kiki@gilmartinir.com
Media Contact:
Meghan Bianco
Taft Communications, a division of RF|Binder
609-544-5446
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5cc5c1f2-5c02-4e0c-82a6-3db3f1859116

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

